Piomet
Piomet (Pioglitazone + Metformin HCl) tablet is composed of two active ingredients that help to control blood sugar levels. It works in the proper utilization of insulin, hence lowering blood sugar levels.
This drug is also used together with diet and exercise to improve blood sugar control in adults with type 2 diabetes mellitus.
Package Contains: Each package contains 14 tablets of Piomet.
Composition:
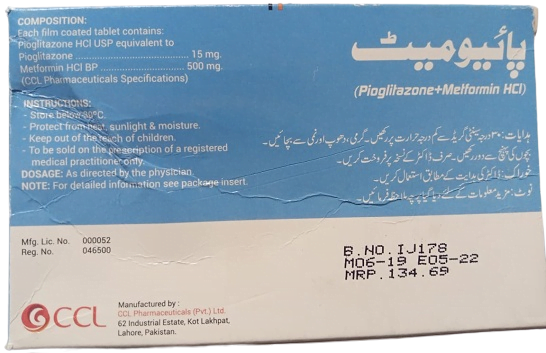
Each film-coated tablet contains:
- Pioglitazone HCl USP equivalent to Pioglitazone 15mg.
- Metformin HCl BP 500mg.
(CCL Pharmaceuticals specifications).
Dosage: As directed by the physicians. Note: For detailed information see package insert.
Precautions:
Pioglitazone should not be administered to patients with type 1 diabetes or those who have diabetic ketoacidosis. It should be used cautiously in combination with insulin, hepatic insufficiency, or heart conditions.
Metformin is known to be excreted largely by the kidney, and the possibility of Metformin accumulation and lactic acidosis increases with renal impairment. Patients with serum creatinine levels higher than the upper limit of normal for their age should not receive this combination.
Side effects:
- Usually, this combination preparation is well tolerated.
- However, with this drug, some patients may feel the visual disturbance and respiratory infections.
- If you experience any of these symptoms, consult your doctor immediately.
Instructions:
- Store below 30°C.
- Protect from heat, sunlight, and moisture.
- Keep out of the reach of the children.
- To be sold on the prescription of a registered medical practitioner only.
Manufacturer & buyer links:
It is manufactured by CCL Pharmaceuticals (Pvt.) Ltd. https://cclpharma.com
You can buy this drug online from: https://nexthealth.pk
By: Tayyaba Zareen